Welcome to the website of Taizhou Longyu Chemical Co., Ltd.!Contact Us |Chinese |English

Taizhou Longyu Chemical Co., Ltd.
10 years of focus on the production of organic peroxides/additives/raw materials
13337792266
Welcome to the website of Taizhou Longyu Chemical Co., Ltd.!Contact Us |Chinese |English

10 years of focus on the production of organic peroxides/additives/raw materials
13337792266
NEWS CENTER
CONTACT US

Time:2022-08-31source:Taizhou Longyu Chemical Co., Ltd
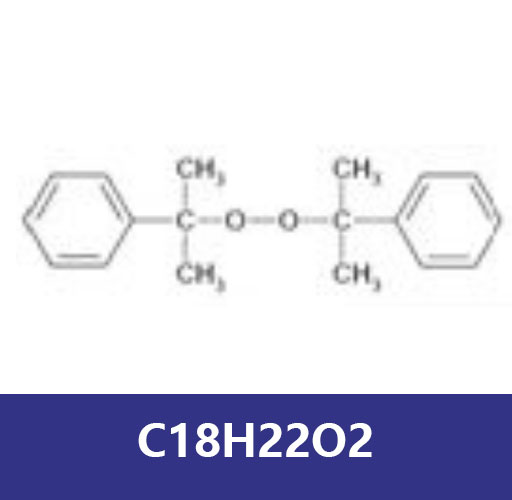
The most important indicator of an initiator's activity is its half-life, t 1/2, which is the time it takes for half of the initial initiator to decompose at a given temperature. For most initiators, the first-order decomposition kinetics are correct, and the half-life is given by: t 1/2 = ln 2 / k d Benzoyl peroxide The most important organic peroxide initiators to date are Benzoyl Peroxide (BPO). It consists of two benzoyl groups bridged by a peroxide bond. BPO is prone to symmetric fission (homogenous) to form two benzoyloxy groups: 2-4 benzoyl peroxide as initiator Some reaction formula The two fragments with unpaired electrons are called free radical initiators. After the free radicals are generated, the free radicals are initiated to react with the monomer units, resulting in a growing polymer chain. The reactivity of BPO is solvent and monomer dependent. In the absence of accelerators, the decomposition rate of diacyl peroxides is first-order, i.e. it is proportional to the initiator concentration: 4,5 R I = d[I ] / d t = -2 d[I] / d t = 2 fk d [I] after integration [I] = [I] 0 exp [ -fk d t ] where f is the initiation efficiency, defined as 4 f = polymerization initiation rate / 2 x initiator decomposition rate in general , the decomposition rate of peroxides in various solvents decreases sequentially: highly halogenated aliphatic < aromatic < most aliphatic < ethers and alcohols < amines. The role of substituents in one or both of the aromatic rings has been extensively studied. As one would expect, the dissociation rate for electron donating groups increases while that for electron withdrawing groups decreases, because the increase in electron density on the oxygen atom increases the repulsive force between them and therefore the OO bond the spontaneous cleavage rate. The presence of amines greatly facilitates the decomposition of benzoyl peroxide. Particularly effective are tertiary aromatic amines, such as dimethylaniline, which accelerate peroxide decomposition by single electron transfer: 6,7 A similar one electron transfer is observed for chloride ions (CL- or ferrous ions (Fe) 2+:) Reaction with peroxide 6 Di-tert-butyl peroxide (DTBP) is another important free radical initiator, mainly used to initiate LDPE polymerization and cross-link elastomers ( such as silicone and EPDM). It is particularly suitable as an initiator because its thermal decomposition temperature is above 100°C, so it is much higher than most other initiators. Although there are several other initiators with higher decomposition temperatures, such as aliphatic dialkyl peroxides, DTBP is usually preferred due to its lower explosion hazard and therefore easier handling. It can also be easily purified.